Conforming To Experimental Requirements
Each experiment has a number of requirements which all of its simulations are expected to conform to. For example, an experiment may have the requirement that all simulations must start and end on particular dates, i.e. a temporal constraint. The full set of experimental requirements for each experiment within a MIP can be explored in the ES-DOC documentation viewer.
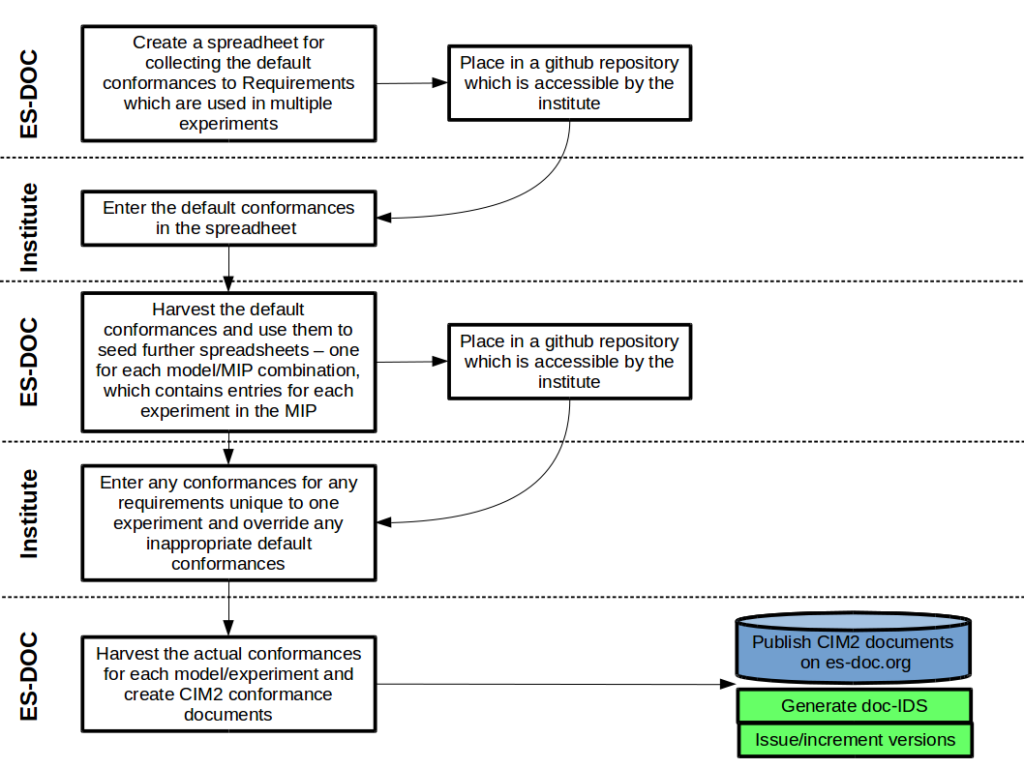
It is essential to document how these requirements were met for each experiment’s ensemble. Generally speaking it is only necessary to state once how a requirement was conformed to, regardless of how many different experiments use that requirement. For example, the requirement which specifies pre-indiustrial carbon dioxide concentration is widely re-used across many experiments and many MIPs, but how this was achieved is likely to be the same in all cases so it needs only to be described once. If there are any exceptions to the general conformance of a requirement, the default “general” answer may easily be overridden.
A conformance description has three components:
1. Whether or not the requirement was met ?
2. How any input datasets were modified ?
3. Additional information ?
Note that components 1 & 2 are necessary only if needed by the requirement .
ES-DOC will initially provide to the groups pre-seeded spreadsheets with which to record the default conformance to each experimental requirement. Subsequent spreadsheets will be provided for overriding the default conformance descriptions for any particular model/experiment combination.